– Wendy Adams, Research Grant Director, Bay Area Lyme Foundation
This pandemic has brought many different modalities in diagnostics, drug development and vaccines to the popular press. In the Tick-borne Disease (TBD) community, we have seen the issues that arise when the timely diagnosis and treatment of infectious disease are hampered by insensitive diagnostics and ineffective treatments.
It bears repeating however, that drugs that fight the infection in question (antibiotics, antiparasitics, or antivirals) are a large part of any eventual solution to an outbreak, especially in advance of a vaccine (see HIV). Antimicrobial therapeutics help keep the pathogen from replicating uncontrolled, allowing the complicated immune system processes to catch up to it, control it and then eradicate it.
One specific treatment modality is being widely discussed: monoclonal antibodies (mAbs). These are the drugs upon which the whole biotech industry and companies like Genentech, Biogen and Amgen were literally built. Six out of the top 10 drugs by sales are mAbs, mostly for oncology and autoimmune disease indications. However, mAbs have not been commonly used for infectious disease (with one major exception we’ll talk about later).
What are monoclonal antibodies? How do they work?
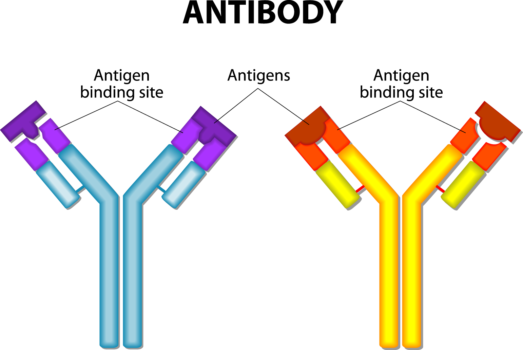
Antibodies are proteins made by the mammalian immune system. They are a workhorse of the acquired immune response and fight specific antigens, which can be anything from an invading pathogen to an aberrant cell or cytokine that needs destruction. Monoclonal antibodies as a drug class are also very specific and only bind to one antigen. They can bind to a single receptor on the outside of a cell, so that cell can’t receive or send out a message. Or the cell can be tagged so the immune system recognizes the cell as foreign and can destroy it. Binding only one target is important to reduce side effects caused by binding to multiple targets.
The largest selling drug in the US, Humira™ (adalimumab), is a mAb that binds to tumor necrosis factor alpha (TNF-a), a cytokine, to keep this chemical messenger from overstimulating inflammation in autoimmune diseases like rheumatoid arthritis or Crohn’s disease. Besides therapeutics, mAbs are widely used as reagents in the research lab and in commercial diagnostics, including the first tier Lyme ELISA.
MAbs aren’t used for all clinical indications for good reasons. Their large size means they can’t get through the cell membrane to act inside a cell, where many drugs need activity. They can’t be dosed orally—because as proteins, mAbs would be seen as food by the stomach and broken down by the digestive process before reaching the bloodstream. Instead, they are given by IV infusion or subcutaneous injection at home every few weeks. They are incredibly expensive to manufacture because they have to be “brewed” or grown up in large bioreactors using recombinant cell lines. (Fun fact: this “brewing” process is used in manufacturing the heme that makes the Impossible Burger taste meaty.)
MAbs have not been used widely for infectious disease. One notable exception is a drug called Synagis ® (palivizumab), which is a prophylactic mAb used in premature infants and young children under the age of 2. Synagis is a manufactured version of the human antibody against respiratory syncytial virus (RSV), an infection that can be serious or fatal in these young children. Instead of a vaccine being given to induce the immune system to manufacture certain antibodies, Synagis delivers the already made antibodies directly into the bloodstream. Synagis gives the immune system a headstart in fighting the virus before the immune system has matured. More recently, antibodies have been used to treat Ebola virus.
Use in COVID-19
For the SARS-CoV2 virus (coronavirus), monoclonal antibodies are being investigated for use in a few different ways. First, there’s the effort to use monoclonal antibodies directly against the virus, just like Synagis. With a new disease like Covid-19, this process of determining which antibodies are protective against the virus is just getting underway. Several biotech companies, like Vir Biotech and Regeneron, are working on these antibodies right now. Treating very ill patients with plasma from recovered patients is a stop gap measure that uses the same mechanism—to give the immune system more ammunition to fight the disease. In addition, there are other drugs to target the spike protein on the coronavirus which allows the virus to enter the cell. Using mAbs to block the binding of the virus to the cell inhibits the virus entering and overtaking the cell to make more copies of the virus. Here is a list of current drug candidates being developed or tested for COVID-19 disease.
Another approach to treating COVID-19 disease is to mitigate the “cytokine storm” that can develop later in the course of infection. This serious, sometimes fatal condition occurs when the immune response goes awry after infection or after administration of certain drugs, and the manufacturing process of new white blood cells goes into hyperdrive. This approach mimics the Humira™ strategy because the body’s own immune response, not a pathogen, is being targeted. Indeed, many of the drugs being evaluated for cytokine storm in COVID-19 are either currently marketed, or in clinical trials, for autoimmune disease.
For other infectious diseases, the coronavirus effort could harken a new age in antimicrobial development. Antibiotics were historically developed with the goal to address a whole class of bacteria—gram-positive or gram negative—and that broad activity was seen as a distinct advantage. With major concerns about antibiotic resistance, harm to the microbiome and a dearth of government investment in novel antibiotic strategies, bringing private industry back into targeted antimicrobial development could be important to a new generation of treatments of pathogens beyond antivirals.
Switching to Lyme disease therapeutics, how can we deploy the specificity of monoclonal antibodies for Lyme and potentially other tick-borne disease? Prophylactic mAbs are already being evaluated for Lyme disease—against OspA. Mark Klempner MD of UMASS is developing OspA antibodies to be delivered at the beginning of each season, using the same mechanism as Synagis. Unlike Synagis, however, these antibodies are not sufficient to prevent disease in humans and have to travel to the feeding tick to kill B. burgdorferi. In addition, the mAbs have to circulate for months to maintain activity throughout the majority of the Lyme season (and we know that season is ever-expanding, and in certain places year-round). Getting large molecules like antibodies to circulate in the body for so long is also not easy, although Dr. Klempner has reportedly solved that problem. Unfortunately, targeting OspA exclusively precludes efficacy against any other tick-borne infection, including B. miyamotoi, as relapsing fever spirochetes don’t have any OspA to target.
Finding other monoclonal antibodies to target B. burgdorferi is something Bay Area Lyme Foundation (BAL) has been supporting through work in multiple labs. Bill Robinson, MD, PhD at Stanford, a member of BAL’s Scientific Advisory Board (SAB), oversees a large effort evaluating the immune response of several diseases and is also co-founder of Atreca, a publicly-traded antibody company. Dr. Robinson and his post-doctoral scholar Lisa Blum, together with BAL SAB members John Aucott MD and Monica Embers PhD, characterized the immune response in Dr. Aucott’s Lyme patients in a 2018 study in Frontiers in Immunology. They found that the immune response (specifically plasmablast production and antibody development) was less robust in people who developed post treatment Lyme disease syndrome (PTLDS) than in people who returned to health after acute treatment with 21 days of doxycycline. Dr. Blum then isolated the antibodies that did bind B. burgdorferi and variants of these antibodies and additional mAbs are currently being tested in animal models in Dr. Embers’ Tulane lab. These findings are significant, as they point to an underdeveloped and misdirected immune response as a potential cause of PTLDS and Persistent Lyme Disease (PLD).
Monoclonal antibody treatments have revolutionized the treatment of oncology and autoimmune disease, but they have not had the same impact on treating infectious disease. Hopefully the enormous resources rightly being marshalled to fight coronaviruses can eventually be leveraged for other infectious disease, including Lyme disease, when this current pandemic is no longer an immediate threat to us all.
Postscript—on April 16th, Gilead Sciences reported preliminary positive compassionate use data of its drug remdesivir in treating COVID-19. This drug is a nucleoside analog small molecule given by IV and works by inhibiting viral RNA polymerase and thus viral replication. It was previously being evaluated for Ebola virus infection, although it was not approved for that indication. Additional data, including data from randomized controlled trials, are expected in the next several weeks. You can read more about the ongoing trials on remdesivir here.
Steve…I, too, received Regeneron monoclonal infusion for Covid 19. I have rheumatoid arthritis and was amazed how my symptoms of arthritis abated following treatment. I did not anticipate this connection. Unfortunately after a short period of time my pain returned. Hoping you can provide me with an update.
So my question is is Regeneron a great treatment for Lyme disease and coinfection’s and if so will it be available to us and where I live in California Tulare California
Kris, I am not a physician or expert by any means. I received a dose of REGEN-COV (casirivimab and imdevimab) by Regeneron a week ago after testing positive for COVID. Within 36 hours of the infusion my COVID symptoms were eliminated. That is, in and of itself, remarkable. What is more remarkable to me is how much better I feel in general. I was diagnosed and treated for Lyme in 2015/16. I experienced brain fog, fatigue, inflammation in joints and muscles, trouble sleeping with my Lyme. After my antibiotic treatments, I felt somewhat better but still deal with same basic symptoms continually since then. Anecdotally, I can say that I feel better this week in every respect than I have in at least 6 years. While correlation does not equal causation… I can tell you that I immediately started scouring the internet for information about treating post-treatment Lyme disease syndrome (PTLDS) with mAbs. That is what led me to this article and ultimately your posted question. If this reprieve of symptoms continues… it will be a life-changer for me. Best of luck.